Enhanced TDS
Identification & Functionality
- Food Ingredients Functions
- Pharma & Nutraceuticals Functions
- Country of Origin
- Taiwan
- Technologies
- Product Families
Features & Benefits
- Benefit Claims (Health)
- Labeling Claims
- Food Ingredients Features
- Product Highlights
- Specific probiotic strains naturally originate from humans and conventional food.
- All the needed nutrients in one
- Multi-use end product in food and beverage
- Thermostable
- Create a positive environment for the colonization and growth of probiotics
- Promote the growth of other beneficial bacteria e.g. AKK bacteris, lactobacillus, and bifidobacteria (Prebiotic Properties)
- Contains multiple enzymatic substances to aid digestion
- Promote gastrointestinal motility and alleviate constipate
- Stimulate the production of anti- inflammatory factors such as IL-10.
Applications & Uses
- Markets
- Applications
- Dosage Form
- Food & Nutrition Applications
- Usage Information
Suggested Volume % in Different Food Products
Test item PE0401 suggested amount %
Cookies 1~2% bakery 1~2% Ice cream 1-2% Chocolate 1~3% Jam 1~3% Seasoning in instant soup/noodle 10~20% Frozen food 0. 1~0.5%
Properties
- Physical Form
- Odor
- Characteristic odor
- Appearance
- White to light brown powder
- Chemical Properties
Value Units Test Method / Conditions pH (Measured as 10% Solution) max. 5 - GB 5009.238 - Typical Properties
Value Units Test Method / Conditions Water Activity max. 0.6 aw GB 5009.3 Moisture Content max. 7 % - - Microbiological Values
Value Units Test Method / Conditions Yeast & Molds Count max. 100 CFU/50g GB 4789.15 Total Aerobic Microbial Count max. 100 CFU /g P-02-19 (in house) Staphylococcus Aureus Negative CFU/25g - Salmonella Negative CFU/25g - Listeria monocytogenes Negative CFU/25/g - Escherichia Coli Negative CFU/50g - Coliforms Negative MPN/50g - - Composition
Value Units Test Method / Conditions Maltodextrin 16.5 % - Lactobacillus Salivarius Fermentation Metabolites 16.5 % - Lactobacillus Plantarum Fermentation Metabolites 16.5 % - Lactobacillus Acidophilus Fermentation Metabolites 16.5 % - Isolated Soy Protein 17 % - Glucose 0.5 % - Bifidobacterium Longum Fermentation Metabolites 16.5 % - - Specifications
Value Units Test Method / Conditions Lactic Acid min. 5 g/100g HPLC (in house) - Amino Acids Composition
Amino acids Concentration (mM) Ratio ALA (Alanine) 34.69 11.64 % LEU (Leucine) 24.34 12.03 % GLU (Glutamine) 20.63 11.44 % VAL (Valine) 15.12 6.67 % ASP (Aspartic acid) 10.54 5.29 %
Regulatory & Compliance
- Certifications & Compliance
- FDA Disclaimer
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
Technical Details & Test Data
- Organic Acids Test Data
Totipro® PE0401 riches in Organic Acids
Fermented lactate (L-form) analysis
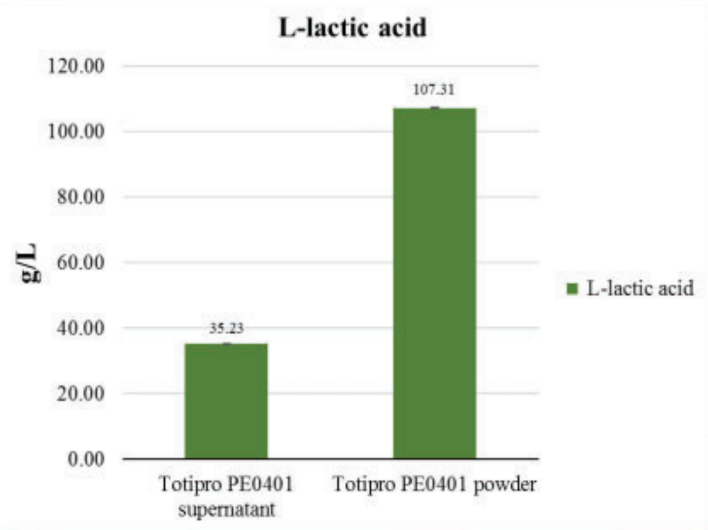
Applied colorimetric method to detect the concentrate of L- Lactic acid of Totipro PE0401- Totipro® PE0401 Test Data
Totipro® PE0401 Inhibit H. Pylori to Support Stomach Health
Methods: incubated H. pylori with 1% Totipro® PE0401 to analyzed the survival rate of H. pylori and urease activity in the broth to evaluate the capacity of against H. pylori.
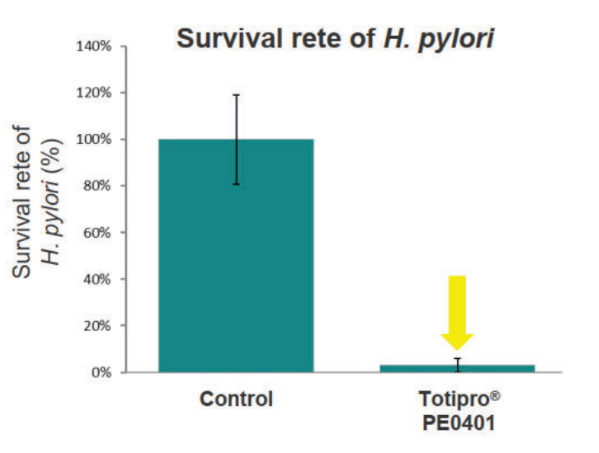
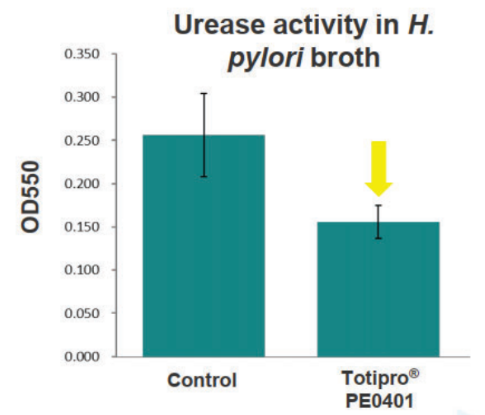
- Anti-oxidant Capacity Test Data
Totipro® PE0401 has Anti-oxidant Capacity to Improve Individual Health
Oxidative stress is the causes of many diseases, which is an imbalanced oxidative metabolic status caused by free radicals. Free radical is a byproduct generated by metabolism in our body and it is playing certain physiological functions, such as signal transduction, defense, and etc.
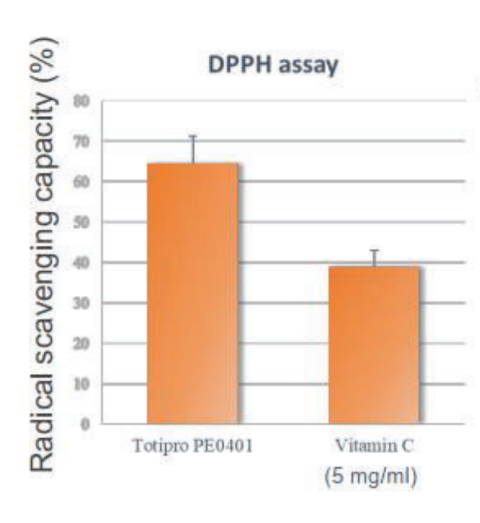
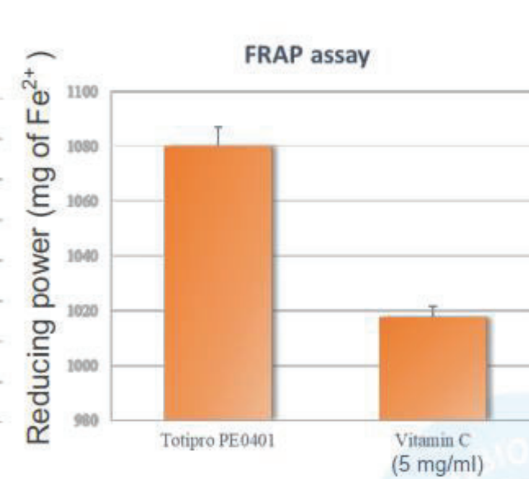
Totipro® PE0401 has high SOD-like activity and have high capacity of radical clearance and reductive ability- Totipro® PE0401 Relieved Diarrhea
Method: The animal model was using 28 days old LYD weaned piglets. Feeding weaned pigs general feed were prone to diarrhea. Added 0.2%, 0.1% Totipro® PE0401, or combined with probiotics into general feed to observe diarrhea rate weekly.
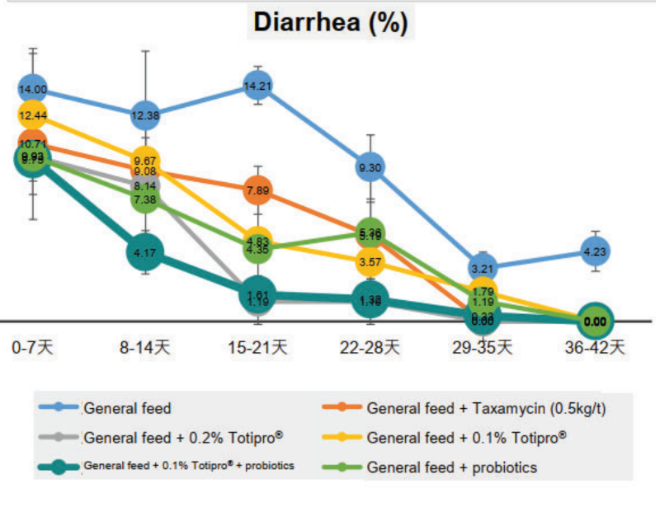
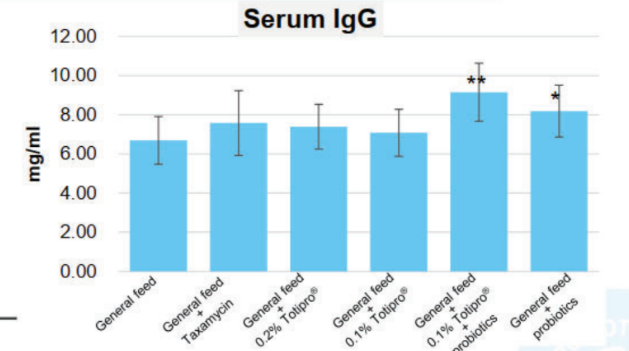
Totipro® PE0401 could improve diarrhea and enhance immunity, which is recommended to supplement with probiotics
- Test Data
PE0401 Improved Gut Microbiota
Methods: 45 subjects, randomly assigned in placebo, low dose Totipro™ PE0401 (200mg/day), and high dose Totipro™ PE0401 (600mg/day). Daily took 3 times to reach testing dose for 30 days. In the final week, analyzed bowel movements, gastrointestinal symptoms, NGS fecal bacteria analysis, and blood biochemical tests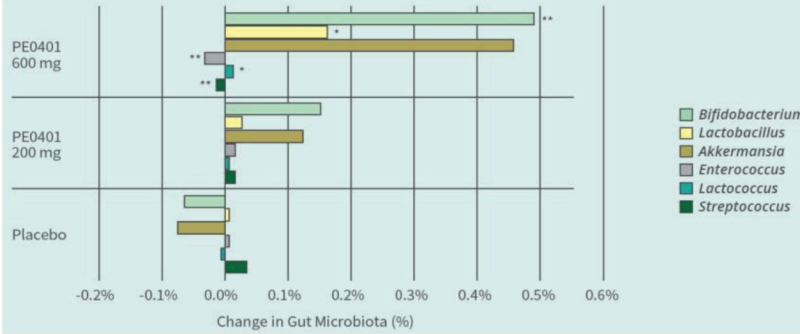
PE0401 increased beneficial bacteria and decreased potential harmful bacteria
- PE0401 Regulated Physiological Functions
Methods: 45 subjects, randomly assigned in placebo, low dose Totipro™ PE0401 (200mg/day), and high dose Totipro™ PE0401 (600mg/day). Daily took 3 times to reach testing dose for 30 days. In the final week, analyzed bowel movements, gastrointestinal symptoms, NGS fecal bacteria analysis, and blood biochemical tests
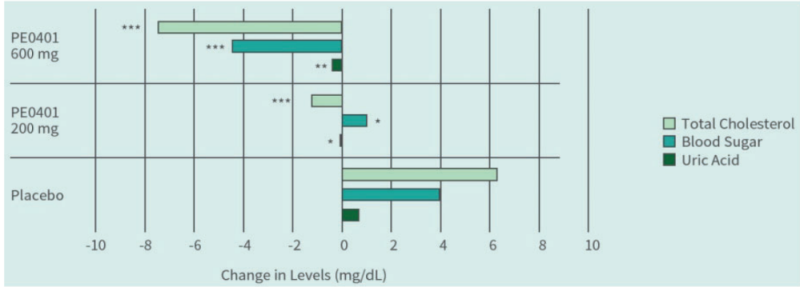
PE0401 helped control fasting blood glucose, uric acid, and total cholesterol levels
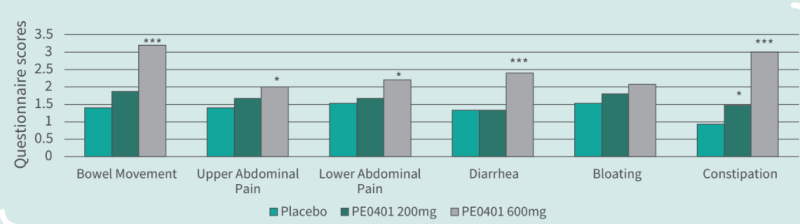
- Clinical Study
Clinical Study on 45 subjects with issues on bowel movements, conducted for 4 weeks, with 15 subjects in control group (placebo capsules containing maltodextrin), 15 subjects in low dose group (capsules containing Totipro™ PE0401, 200 mg/day), and 15 subjects in high dose group (capsules containing Totipro™ PE0401, 600 mg/day) (* p < 0.05, ** p < 0.01, *** p ≤ 0.001, vs. control)
- Totipro™ PE0401 may support digestive health, such as bowel movement, and relieve abdominal pain, diarrhea, and constipation
Packaging & Availability
- Packaging Type
- Packaging Information
1 kg / 5 kg / 20 kg per aluminum foil bag
Storage & Handling
- Shelf Life
- 3 years
- Storage and Shelf Life Conditions
- Keep dry and cool, preferably below 25°C. Avoid sun light, moisture and high temperature.
- Shelf Life 3 Years

