Enhanced TDS
Identification & Functionality
- Active Component
- Ingredient Name
- Pharma & Nutraceuticals Functions
- Technologies
- Product Families
Features & Benefits
- Benefit Claims (Health)
- Labeling Claims
- Food Ingredients Features
- Product Highlights
- 100,000X aqueous solubility
- 3.7X oral bioavailability
- Scientifically proven efficacy
- Unique absorption mechanism
- 2023 US FDA New Dietary Ingredient
- Proven Efficacy
Applications & Uses
- Markets
- Applications
- Dosage Form
- Food & Nutrition Applications
- Use Level
- 40 - 80 mg
Properties
- Physical Form
- Soluble In
- Appearance
- White to pale yellow fine powder
- Chemical Properties
Value Units Test Method / Conditions Water-soluble Isoflavone Derivatives min. 70 % QA_Q02_AH1,HPLC Arsenic Content max. 2 ppm MOHWH0014.03 - Physical Properties
Value Units Test Method / Conditions Loss on Drying max. 6 % QA-Q01-AH1 - Microbiological Values
Value Units Test Method / Conditions Yeast & Molds Count max. 102 cfu/g MOHWM0008.01 Total Aerobic Plate Count max. 103 cfu/g MOHWM0014.01 Salmonella Negative /25g MOHWM0025.01 Escherichia Coli Negative /g MOHWM0023.02 Enterobacteria max. 10 cfu/g MOHWM0028.00 - Heavy Metals
Value Units Test Method / Conditions Lead Content max. 20 ppm MOHWH0014.03
Regulatory & Compliance
- Certifications & Compliance
- FDA Disclaimer
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
Technical Details & Test Data
- Pharmacokinetic Profile
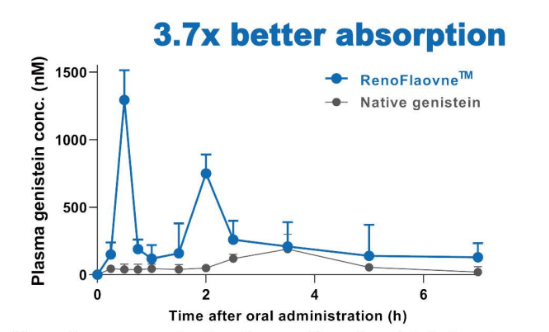
Mean plasma concentration-time profiles of genistein in rats after oral adminstration of native genistein and RenoFlavone™ at 3.7 u mol/kg bw. Data are mean SE (n = 4).- Animal Study Improve Menopausal Bone Health
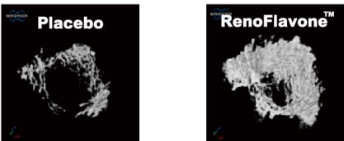
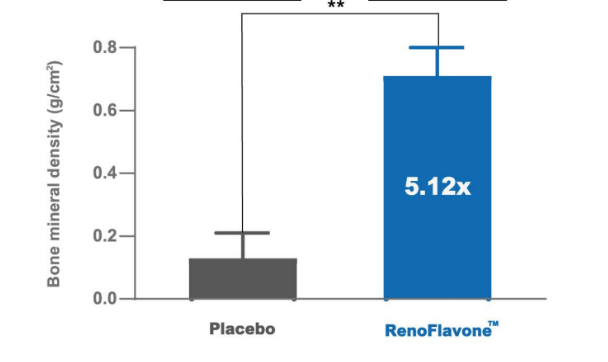
Osteoprotective effect of RenoFlavone™, a derivative of genistein with high bioavailability, in ovariectomized rats. Mi ovarietomized rats. Bet of plate and entral met wip (15.2 umol/kg bw/day) after 12-week treatment. Data are mean ‡SD, n = 6. Value are mean‡SEM. ** p < 0.01. RenoFlavone™ vs placebo group (paired t-test).- Human study Relieve menstrual discomfort
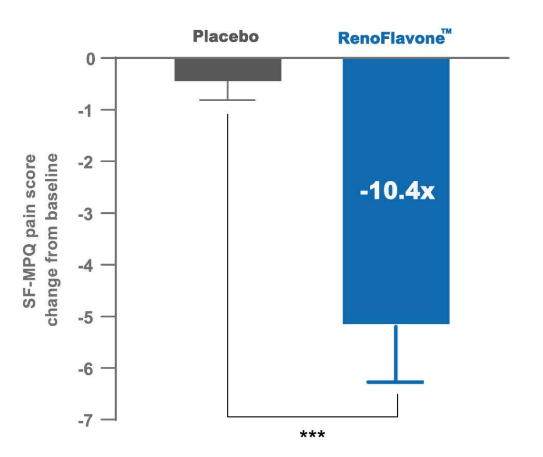
A randomized controlled trial of RenoFlavone™ in the treatment of primary dysmenorrhea. Effects of RenoFlavone™ (20 mg/ day) and placebo on menstrual pain score (Short form McGill pain questionnaire score, SF-MPQ) in first month (The trial is ongoing). Value are mean SEM, n = 40. Comparison of changes between the study groups (chi-square test). Significance of double star symbol is *** p< 0.001.
Packaging & Availability
- Packaging Type
Storage & Handling
- Shelf Life
- 3 years
- Storage and Shelf Life Conditions
Store in aluminum bag at controlled room temperature for 3 years.

