Enhanced TDS
Identification & Functionality
- Chemical Name
- Pharma & Nutraceuticals Functions
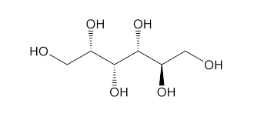
- Molecular formula
- C₆H₁₄O₆
- Technologies
- Product Families
- Definition
Sorbitol contains not less than 91.0% and not more than 100.5% of D-sorbitol (C₆H₁₄O₆), calculated on the anhydrous basis. The amounts of total sugars, other polyhydric alcohols, and any hexitol anhydrides, if detected, are not included in the requirements, nor in the calculated amount as stated in General Notices, 5.60.10 Other Impurities in USP and NF Articles.
- Chemical Structure
Properties
- Physical Form
- Soluble In
- Typical Properties
Value Units Test Method / Conditions Molecular Weight 182.17 - -
Regulatory & Compliance
- Certifications & Compliance
- Chemical Inventories
- FDA Disclaimer
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
- USP Reference Standards
- USP Sorbitol RS
Packaging & Availability
- Labelling Information
Sorbitol intended for use in preparing parenteral dosage forms is so labeled.
Storage & Handling
- Storage Information
Preserve in well-closed containers. No storage requirements are specified.
