Enhanced TDS
Identification & Functionality
- Chemical Name
- Molecular formula
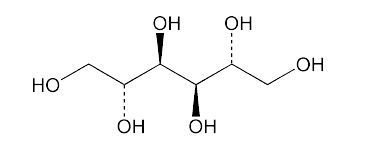
- C₆H₁₄O₆
- Technologies
- Product Families
- Definition
Mannitol contains not less than 97.0% and not more than 102.0% of mannitol (C₆H₁₄O₆), calculated on the dried basis.
- Chemical Structure
Applications & Uses
Properties
- Physical Form
- Soluble In
- Typical Properties
Value Units Test Method / Conditions Molecular Weight 182.17
Regulatory & Compliance
- Certifications & Compliance
- Chemical Inventories
- FDA Disclaimer
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
- USP Reference Standards
- USP Mannitol RS
Packaging & Availability
- Labelling Information
The label states, where applicable, the maximum concentration of bacterial endotoxins. The label states, where applicable, that the substance is suitable for use in the manufacture of parenteral dosage forms.
Storage & Handling
- Storage Information
Preserve in well-closed containers.
