Enhanced TDS
Identification & Functionality
- Chemical Name
- Pharma & Nutraceuticals Functions
- Molecular formula
- C₁₇H₂₁NO · HCl
- Technologies
- Product Families
- Definition
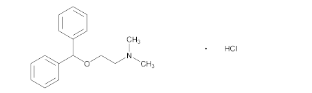
Diphenhydramine Hydrochloride contains not less than 98.0% and not more than 102.0% of diphenhydramine hydrochloride (C₁₇H₂₁NO · HCl), calculated on the dried basis.
- Chemical Structure
Applications & Uses
Properties
- Physical Form
- Soluble In
- Typical Properties
Value Units Test Method / Conditions Molecular Weight 291.82
Regulatory & Compliance
- Certifications & Compliance
- Chemical Inventories
- FDA Disclaimer
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
- USP Reference Standards
- USP Diphenhydramine Hydrochloride RS USP Diphenhydramine Related Compound A RS 2-(Diphenylmethoxy)-N-methylethanamine hydrochloride
- C16H19NO · HCl 277.79
