Enhanced TDS
Identification & Functionality
- Ingredient Name
- Food Ingredients Functions
- Pharma & Nutraceuticals Functions
- Technologies
- Product Families
Features & Benefits
- Benefit Claims (Health)
- Labeling Claims
- Food Ingredients Features
Applications & Uses
- Markets
- Applications
- Dosage Form
- Food & Nutrition Applications
- Use Level
- 5 × 10⁹ cfu/g
Properties
- Physical Form
- Appearance
- White to light brown color powder
- Microbiological Values
Value Units Test Method / Conditions Yeast & Molds Count max. 100 cfu/g Taiwan Food and Drug Administration ( TFDA ) method Staphylococcus Aureus Negative cfu/g Taiwan Food and Drug Administration ( TFDA ) method Salmonella Negative cfu/g Taiwan Food and Drug Administration ( TFDA ) method Listeria Monocytogenes Negative cfu/g Taiwan Food and Drug Administration ( TFDA ) method Eschericha Coli Negative cfu/g Taiwan Food and Drug Administration ( TFDA ) method Coliforms Negative cfu/g Taiwan Food and Drug Administration ( TFDA ) method - Specifications
Value Units Test Method / Conditions Water Activity max. 0.25 aw Taiwan Food and Drug Administration ( TFDA ) method Viable Cell Counts max. 3.0 × 10¹¹ cfu/g Taiwan Food and Drug Administration ( TFDA ) method Moisture Content max. 7 % Taiwan Food and Drug Administration ( TFDA ) method - Composition
NO. Composition Content (%) 1 Lactobacillus rhamnosus MP108 100% Total 100%
Regulatory & Compliance
- Certifications & Compliance
- FDA Disclaimer
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
Technical Details & Test Data
- Product Characteristics
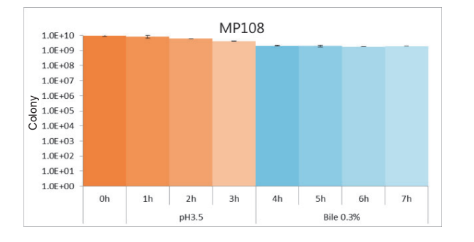
High Gastric Acid and Bile Tolerance:
MP108 remained high bioavailability in the simulated environment with Gastric Acid and Bile for 7 hours.
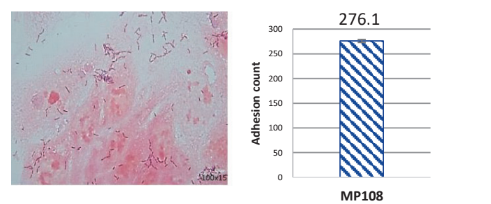
Strong Colonization on Caco-2 Intestine Cells:
MP108 highly adheres to Caco-2 intestine cells
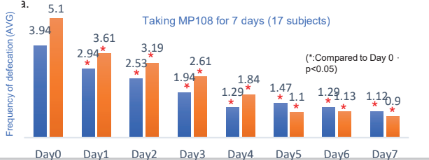
Improve Diarrhea and Constipation:
Infants and young children suffering diarrhea (>3 times a day) were given MP108 ( 5×109CFU/day) (blue bar) or MP108 probiotic combined with antibiotics (orange bar) for 7 days, and the result shows that MP108 can improve the symptoms of diarrhea.
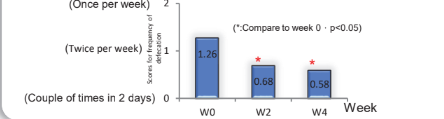
80 subjects with severe diarrhea ( Less than 3 times of defecation) took MP108 ( 10 billion cfu/day), administration 4 weeks.
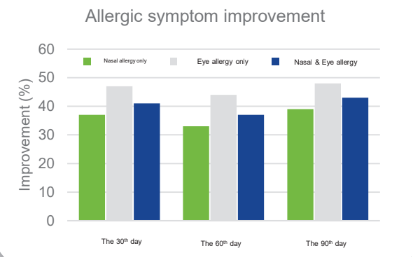
Improve Allergic Symptoms:
Clinical Trial : 59 subjects aged 6-13 years old with Chronic rhinitis took MP108 ( 5 billion cfu/day) with antihistamine for the first 30 days, and stop taking antihistamine during the following 60 days, but MP108 alone.
Improve Atopic Dermatitis:
Clinical Trial: 66 infants aged 4-48 months with dermatitis took MP108 (5 billion cfu/day) and applied topical corticosteroids. Administration 8 weeks.
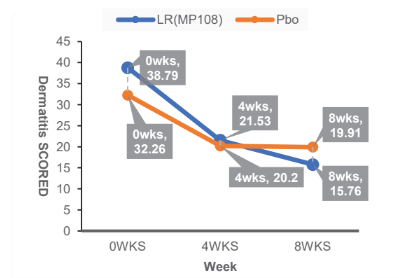
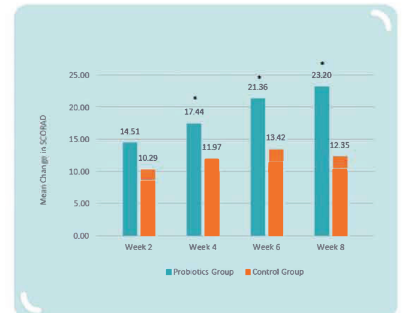
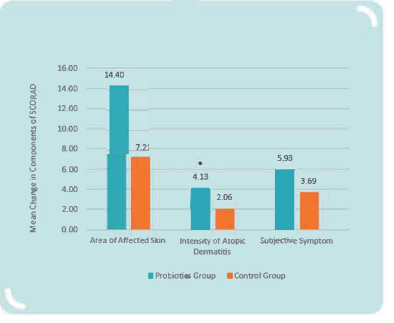
Clinical Study: on 62 infants and young children aged 4-48 months and diagnosed with atopic dermati with a Scoring of Atopic Dermatitis (SCORAD) ≥ 15, conducted for 8 weeks, with 32 subjects in control group (placebo) and 30 subjects in probiotics group (MP108) (* p < 0.05 vs. control group):
MP108 could improve atopic dermatitis:
MP108 could reduce the intensity of atopic dermatitis
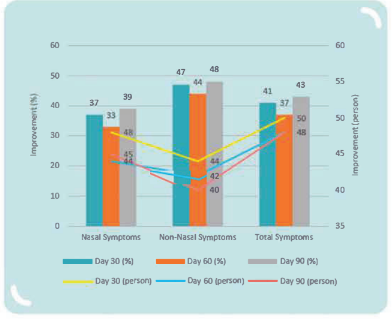
Clinical Study on 59 children aged 6-13 years and diagnosed with allergic rhinitis for > 1 year with medium to severe symptoms, conducted for 90 days, with administration of antihistamine therapy and MP108 capsules during the first 30 days and MP108 capsules only during the other 60 days:
MP108 helps improve symptoms of allergic rhinitis
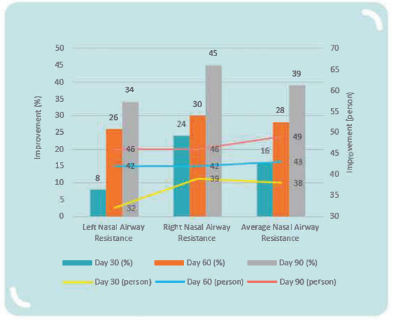
MP108 helps reduce nasal airway resistance
Packaging & Availability
- Packaging Type
- Packaging Information
1kg a bag with nitrogen filling and vacuum packing.
Storage & Handling
- Shelf Life
- 24 months

