Enhanced TDS
Identification & Functionality
- Ingredient Name
- Food Ingredients Functions
- Pharma & Nutraceuticals Functions
- Technologies
- Product Families
Features & Benefits
- Labeling Claims
- Food Ingredients Features
Applications & Uses
- Markets
- Applications
- Food & Nutrition Applications
Regulatory & Compliance
- Certifications & Compliance
- FDA Disclaimer
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
Technical Details & Test Data
- Product Characteristics
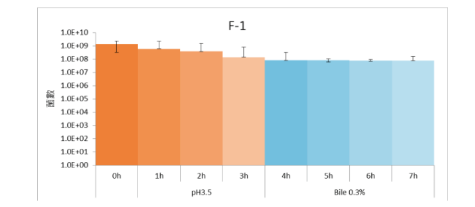
High Gastric Acid and Bile Tolerance:
F-1 remained high bioavailability in the simulated environment with Gastric Acid and Bile for 7 hours
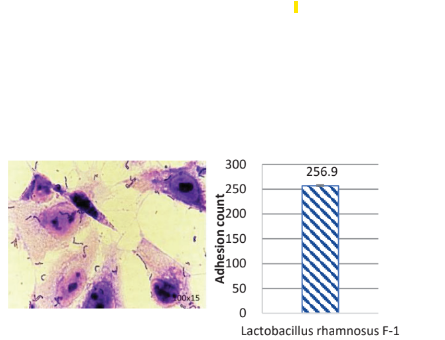
Strong Colonization on Caco-2 Intestine Cells:
F-1 highly adheres to Caco-2 intestine cells
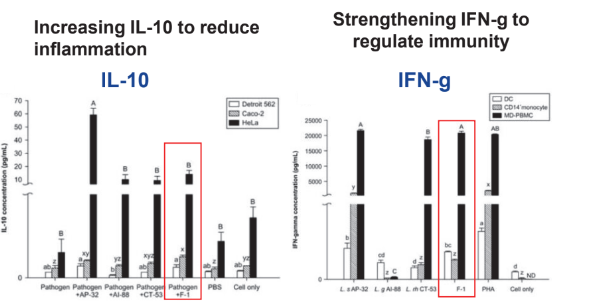
Immunity Regulation and Anti-Inflammation:
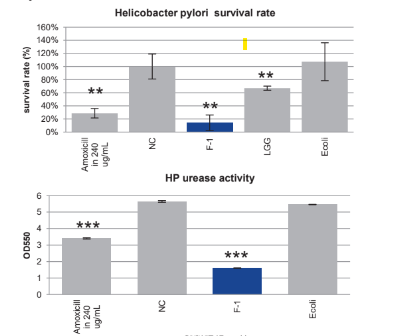
Helicobacter pylori Inhibitory Effect:
In vivo study: After co-culturing with Helicobacter pylori (HP), F-1 can inhibit the growth of Helicobacter pylori (HP), and inhibit the urease activity of HP.
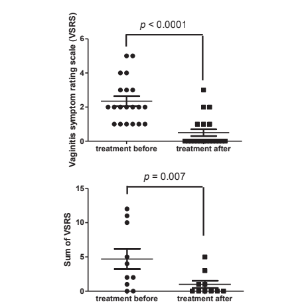
Anti-Vaginitis:
Human clinical trial: 32 subjects with vaginitis took PRONULIFE VagProtect containing F-1 with 5 billion CFU/capsule, twice per day for 4 weeks
Packaging & Availability
- Packaging Type
- Packaging Information
1kg a bag with nitrogen filling and vacuum packing.
Storage & Handling
- Shelf Life
- 24 months
- Stability Test
F-1 remains stable at 4°C and 25°C for 24 months:
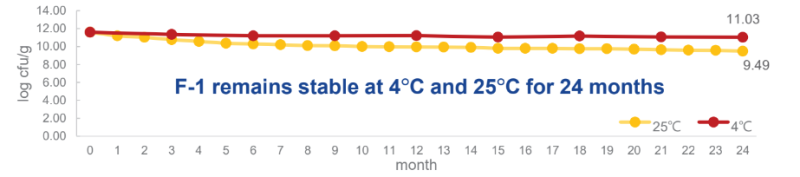
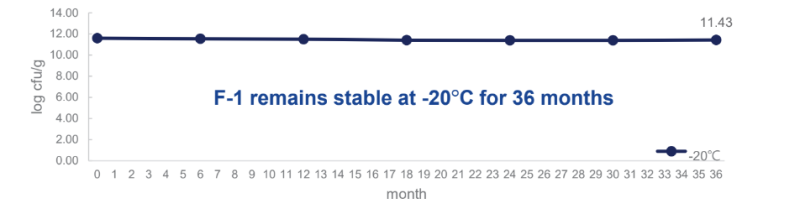
F-1 remains stable at -20°C for 36 months: