Enhanced TDS
Identification & Functionality
- Ingredient Name
- Food Ingredients Functions
- Pharma & Nutraceuticals Functions
- Technologies
- Product Families
Features & Benefits
- Benefit Claims (Health)
- Labeling Claims
- Food Ingredients Features
Applications & Uses
- Markets
- Applications
- Food & Nutrition Applications
Properties
- Physical Form
- Appearance
- White to light brown color powder
- Microbiological Values
Value Units Test Method / Conditions Yeast & Molds Count max. 100 cfu/g Taiwan Food and Drug Administration ( TFDA ) method Staphylococcus Aureus Negative cfu/g Taiwan Food and Drug Administration ( TFDA ) method Salmonella Negative cfu/g Taiwan Food and Drug Administration ( TFDA ) method Listeria Monocytogenes Negative cfu/g Taiwan Food and Drug Administration ( TFDA ) method Eschericha Coli Negative cfu/g Taiwan Food and Drug Administration ( TFDA ) method Coliforms Negative cfu/g Taiwan Food and Drug Administration ( TFDA ) method - Specifications
Value Units Test Method / Conditions Water Activity max. 0.25 aw Taiwan Food and Drug Administration ( TFDA ) method Viable Cell Counts min. 2.0 × 10¹¹ cfu/g Taiwan Food and Drug Administration ( TFDA ) method Moisture Content max. 7 % Taiwan Food and Drug Administration ( TFDA ) method
Regulatory & Compliance
- Certifications & Compliance
- FDA Disclaimer
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
Technical Details & Test Data
- Product Characteristics
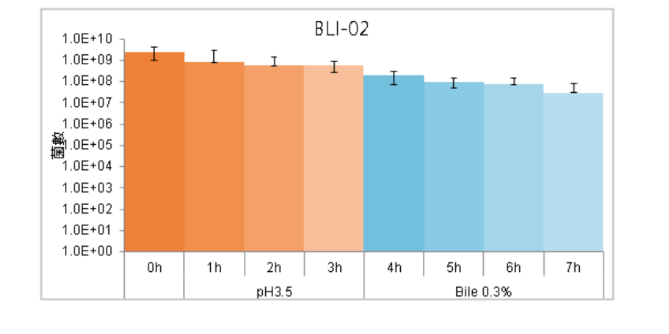
High Gastric Acid and Bile Tolerance:
BLI-02 remained high bioavailability in the simulated environment with Gastric Acid and Bile for 7 hours
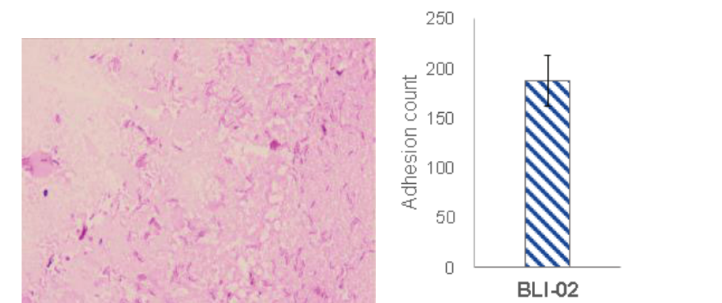
Strong Colonization on Caco-2 Intestine Cells:
BLI-02 highly adheres to Caco-2 intestine cells
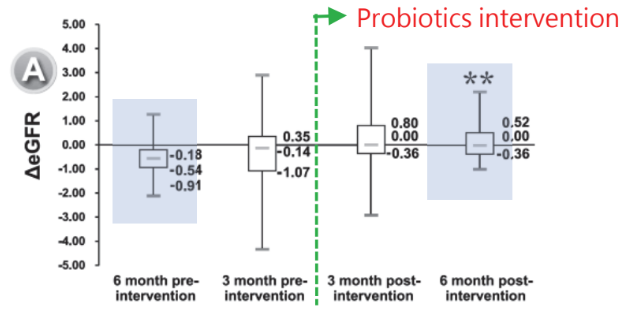
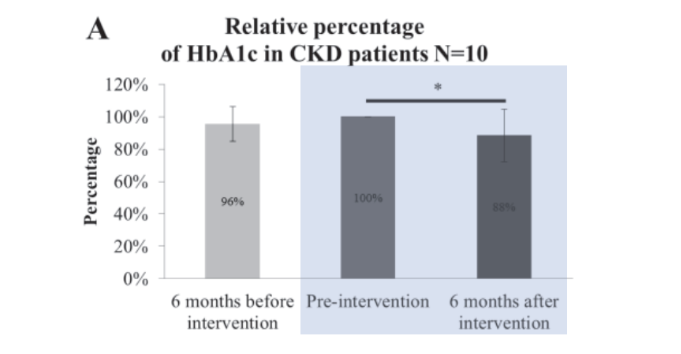
Human clinical trial: 38 Patients with stages 3-5 CKD supplemented with two capsules containing 2.5 billion FU of PRONULIFE® RenaProtect (BLI-02, TYCA06, VDD088) daily for 6 months:
After the 6-month treatment, taking probiotics improved kidney’s function and failure:
RenaProtect helps improve HbA1c level, stabilizing blood sugar:
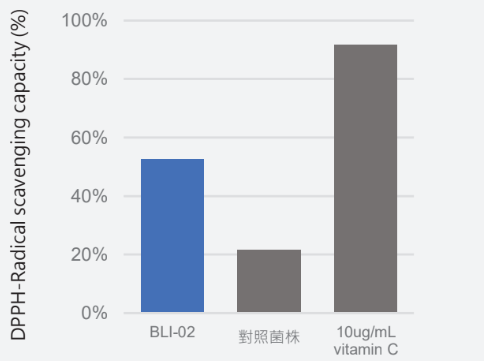
Anti-Oxidant:
Strong ability to eliminate DPPH free radicals
Packaging & Availability
- Packaging Type
- Packaging Information
1kg a bag with nitrogen filling and vacuum packing
Storage & Handling
- Shelf Life
- 24 months
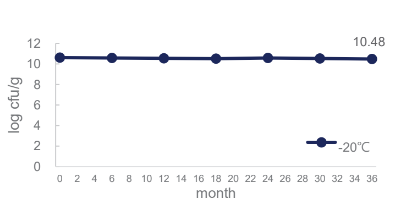
- Stability Test
BLI-02 remains stable at 4°C and 25°C for 24 months:
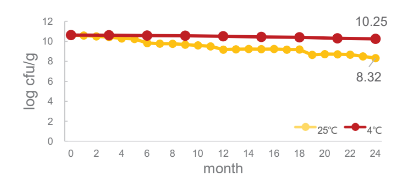
BLI-02 remains stable at -20°C for 36 months: