Enhanced TDS
Identification & Functionality
- Active Component
- Ingredient Name
- Pharma & Nutraceuticals Functions
- Technologies
- Product Families
Features & Benefits
- Benefit Claims (Health)
- Labeling Claims
- Food Ingredients Features
- Product Highlights
- 80,000X aqueous solubility
- 8.2X Superiod Bioavailability
- Scientifically proven efficacy
- Unique absorption mechanism
- Improved the maximum plasma level of Luteolin in systemic circulation by approximately 21.5 times commerical products (2150%)
Applications & Uses
- Markets
- Applications
- Dosage Form
- Food & Nutrition Applications
- Use Level
- 100 - 200 mg
Properties
- Physical Form
- Appearance
- Yellow brown fine powder
- Chemical Properties
Value Units Test Method / Conditions Water-soluble Luteolin Derivatives min. 15 % QA_Q02_AH1,HPLC Arsenic Content max. 2 ppm MOHWH0014.03 - Physical Properties
Value Units Test Method / Conditions Loss on Drying max. 10 % QA-Q01-AH1 - Microbiological Values
Value Units Test Method / Conditions Yeast & Molds Count max. 102 cfu/g MOHWH0008.01 Total Aerobic Plate Count max. 103 cfu/g MOHWH0014.01 Salmonella Negative /25g MOHWH0025.01 Escherichia Coli Negative /g MOHWH0023.02 Enterobacteria max. 10 cfu/g MOHWH0028.00 - Heavy Metals
Value Units Test Method / Conditions Lead Content max. 20 ppm MOHWH0014.03
Regulatory & Compliance
- Certifications & Compliance
- FDA Disclaimer
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
Technical Details & Test Data
- Pharmacokinetic Profile
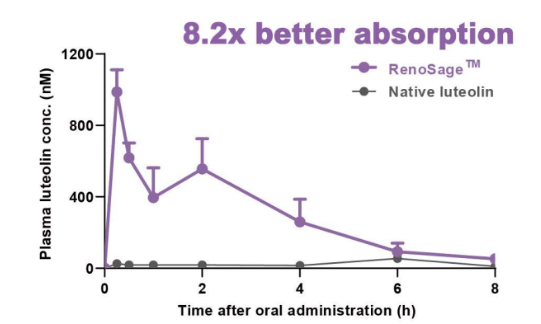
Mean plasma concentration-time profiles of hesperetin in rats after oral adminstration of native hesperidin and RenoCidin™ at 22.8 u mol/kg bw. Data are mean SE (n = 4).- Animal Study Support Cognitive & Memory Health
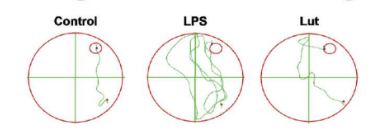
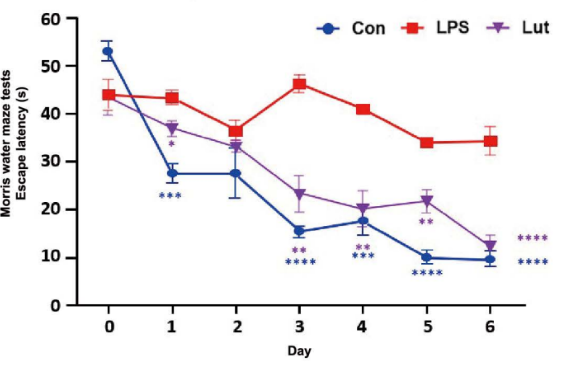
Effects of luteolin on LPS-induced cognitive learning and spatial memory impairment in Morris water maze tests. The swimming and the escape latency of mice during the Morris water maze tests. Mice received luteolin (120 mg/kg/ day) via the intragastric route for 14 days and received LPS (250 pg/kg/day) via an intraperitoneal injection for 7 days before the Morris water maze test started. A two-way ANOVA showed a difference between the Con or Lut vs. LPS (p< 0.0001).
- Human Study Manage Gout and Uric Acid
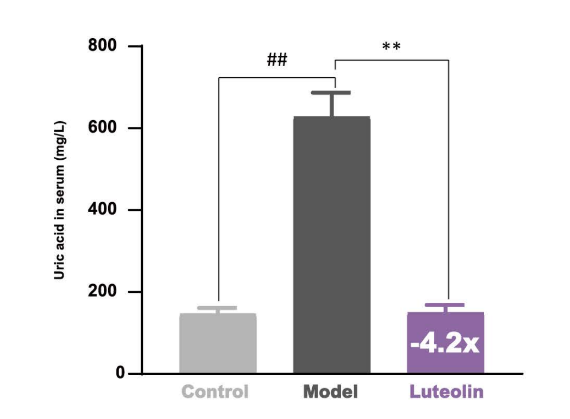
Effects of luteolin on uric acid in serum of potassium oxonate (PO)-induced hyperuricemia mice. Data represent mean ‡ SEM for 10 mice. Mice were administrated with luteolin (100 mg/kg/day for 7 days, n=10) or treated with only vehicle (saline 10 ml/kg/day, n=10) as model group after PO-induced hyperuricemia in mice. ##p< 0.01 compared with normal group: **p< 0.01 compared with model group (independent samples t-test).
Packaging & Availability
- Packaging Type
Storage & Handling
- Shelf Life
- 3 years
- Storage and Shelf Life Conditions
Store in aluminum bag at controlled room temperature for 3 years.

