Enhanced TDS
Identification & Functionality
- Chemical Name
- Molecular formula
- C₁₈H₁₃NNa₂O₈S₂ · H₂O
- Technologies
- Product Families
- Chemical Structure
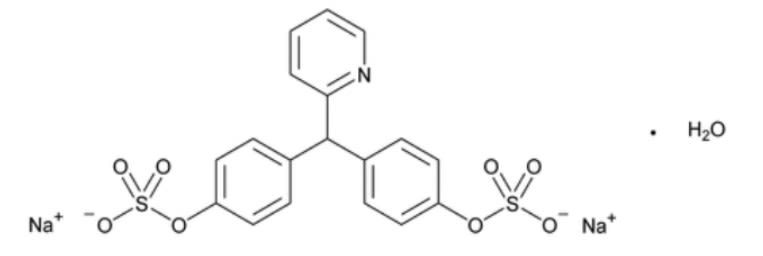
- Chemical Structure
- Defination
Sodium Picosulfate contains NLT 98.0% (USP 1-Aug-2023) and NMT 102.0% (USP 1-Aug-2023) of sodium picosulfate (C₁₈H₁₃NNa₂O₈S₂ ), calculated on the anhydrous basis.
Applications & Uses
Properties
- Typical Properties
Value Units Test Method / Conditions Molecular Weight 499.42 - -
Regulatory & Compliance
- Certifications & Compliance
- FDA Disclaimer
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
- USP Reference Standards
- USP Sodium Picosulfate RS
- USP Sodium Picosulfate Related Compound A RS
- Sodium 4-((4-hydroxyphenyl)(pyridin-2-yl)methyl) phenyl sulfate. C18H14NNaO5S 379.36
Storage & Handling
- Storage Information
Preserve in tight, light-resistant containers. Store at room temperature.
