Enhanced TDS
Identification & Functionality
- Chemical Name
- Pharma & Nutraceuticals Functions
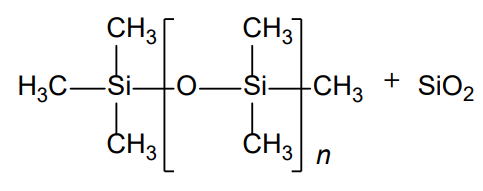
- Molecular formula
- (CH₃)₃Si–[–O-Si(CH₃)₂–]ₙ–CH₃ + SiO₂
- Technologies
- Product Families
- Definition
Simethicone Emulsion is a water-dispersible form of Simethicone composed of Simethicone, suitable emulsifiers, preservatives, and water. It may contain suitable viscosity-increasing agents. It contains an amount of polydimethylsiloxane ([–(CH₃)₂SiO–]n) that is not less than 85.0% and not more than 110.0% of the labeled amount of simethicone.
- Chemical Structure
Features & Benefits
- Labeling Claims
Applications & Uses
Properties
Regulatory & Compliance
- Certifications & Compliance
- Chemical Inventories
- FDA Disclaimer
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
- USP Reference Standards
- USP Polydimethylsiloxane RS
Storage & Handling
- Storage Information
Preserve in tight containers.
