Enhanced TDS
Identification & Functionality
- Chemical Name
- Molecular formula
- C₂₃H₃₂O₆
- Technologies
- Product Families
- Molecular Structure
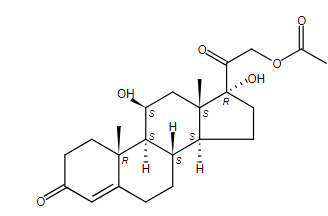
- Definition
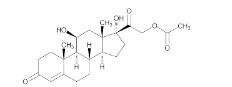
Hydrocortisone Acetate contains not less than 97.0% and not more than 102.0% of C₂₃H₃₂O₆, calculated on the dried basis.
- Chemical Structure
Applications & Uses
Properties
- Physical Form
- Soluble In
- Typical Properties
Value Units Test Method / Conditions Molecular Weight 404.5 - -
Regulatory & Compliance
- Certifications & Compliance
- Chemical Inventories
- FDA Disclaimer
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
Storage & Handling
- Storage Information
Preserve in well-closed containers.

