Enhanced TDS
Identification & Functionality
- Chemical Name
- Molecular formula
- C₁₇H₁₈N₂O₆
- Technologies
- Product Families
- Definition
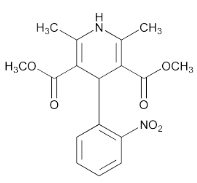
Nifedipine contains NLT 98.0% and NMT 102.0% of nifedipine (C₁₇H₁₈N₂O₆), calculated on the dried basis. [NOTE—Nifedipine, when exposed to daylight and certain wavelengths of artificial light, readily converts to a nitrosophenylpyridine derivative. Exposure to UV light leads to the formation of a nitrophenylpyridine derivative. Perform the Assay and other tests in the dark or under golden fluorescent or other low-actinic light. Use low-actinic glassware.]
- Chemical Structure
Applications & Uses
Properties
- Typical Properties
Value Units Test Method / Conditions Molecular Weight 346.33 - -
Regulatory & Compliance
- Certifications & Compliance
- FDA Disclaimer
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
- USP Reference Standards
- USP Nifedipine RS
- USP Nifedipine Nitropheny|pyridine Analog RS
- Dimethyl 4-(2-nitrophenyl)-2,6-dimethylpyridine-3,5- dicarboxylate. CH16N206 344.33
- USP Nifedipine Nitrosopheny|pyridine Analog R$
- Dimethyl 4-(2-nitrosophenyl)-2,6-dimethy|pyridine-3,5- dicarboxylate. C17H16N2O5 328.33
Storage & Handling
- Storage Information
Preserve in tight, light-resistant containers.
