Enhanced TDS
Identification & Functionality
- Chemical Family
- Chemical Name
- Ingredient Name
- Mineral Type
- Pharma & Nutraceuticals Functions
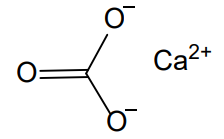
- Molecular formula
- CaCO₃
- Technologies
- Product Families
- Definition
Calcium Carbonate, dried at 200°C for 4 hours, contains calcium equivalent to not less than 98.0% and not more than 100.5% of calcium carbonate (CaCO₃).
- Chemical Structure
Applications & Uses
Properties
- Physical Form
- Appearance
- White gran. powder
- Physical Properties
Value Units Test Method / Conditions Particle Size (Through 16 mesh) 100 % - Particle Size (Through 18 mesh) 100 % - Particle Size (on 60 mesh) 59.45 % - Particle Size (Through 200 mesh) 6.72 % - Tapped Density 1 gm/ml - - Chemical Properties
Value Units Test Method / Conditions Heavy Metals (as Lead) max. 5 ppm - Lead Content max. 0.1 ppm - Cadmium Content max. 0.2 ppm - Mercury Content max. 0.1 ppm - Arsenic Content max. 0.5 ppm - Acid Insoluble (Sub.) 0.08 % - Sulphate Content max. 0.20 % - Chloride Content max. 250 ppm - Iron Content max. 0.002 - - Fluoride Content 0.002 % - Moisture Content 0.48 % - Magnesium Content max. 1.0 % - Assay Content (as Calcium carbonate) 94.73 % - Assay Content (as Calcium) 37.93 % - - Typical Properties
Value Units Test Method / Conditions Molecular Weight 100.09 - Microbiological Values
Value Units Test Method / Conditions Total Aerobic Count max. 10 cfu/gm - Total Moulds & Yeast Count max. 10 cfu/gm - Salmonella Absent /10g USP 37 <2022> E-coli Absent /g - Pseudomonas aeruginosa Absent /g - Staphylococcus aureus Absent /g -
Regulatory & Compliance
- Certifications & Compliance
- Chemical Inventories
- FDA Disclaimer
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
- USP Reference Standards
- USP Sodium Fluoride RS
Storage & Handling
- Storage Information
Preserve in well-closed containers.
