Enhanced TDS
Identification & Functionality
- Active Component
- Chemical Name
- Ingredient Name
- Ingredient Origin
- Food Ingredients Functions
- Pharma & Nutraceuticals Functions
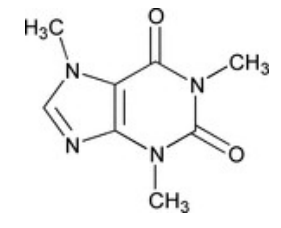
- Molecular formula
- C₈H₁₀N₄O₂
- Technologies
- Product Families
- Chemical Structure
 https://media.knowde.com/image/upload/c_limit,h_800,w_800/v1707719855/production/Ckeditor::Picture/CapturePNG_afnan.tv_2024-02-12T06_37_33.569Z.png.png
https://media.knowde.com/image/upload/c_limit,h_800,w_800/v1707719855/production/Ckeditor::Picture/CapturePNG_afnan.tv_2024-02-12T06_37_33.569Z.png.png
Features & Benefits
Applications & Uses
Properties
- Physical Form
- Soluble In
- Appearance
- White or almost white, crystalline powder or silky white or almost white, crystals.
- Typical Properties
Value Units Test Method / Conditions Molecular Weight 194.19 - - - Microbiological Values
Value Units Test Method / Conditions Total Aerobic Bacteria 800 CFU/g - Yeasts & Molds Count 80 CFU/g - - Specifications
Value Units Test Method / Conditions Acidity max. 0.2 ml - Melting Point 235.0 - 237.5 °C FCC11 Sulfates max. 500 ppm BP 2019. EP 100 Water Determination 0.5 % USP43 Sulphated Ash 0.1 % BP 2019, EP 10.0 - Impurities
Value Units Test Method / Conditions Organic Impurities (Individual impurity) 0.1 % USP43 Organic Impurities (total impurities) 0.1 % USP43 - Heavy Metals
Value Units Test Method / Conditions Heavy Metals max. 10 ppm -
Regulatory & Compliance
- Certifications & Compliance
- Chemical Inventories
- FDA Disclaimer
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
