Enhanced TDS
Identification & Functionality
- Chemical Name
- Pharma & Nutraceuticals Functions
- Technologies
- Product Families
- Definition
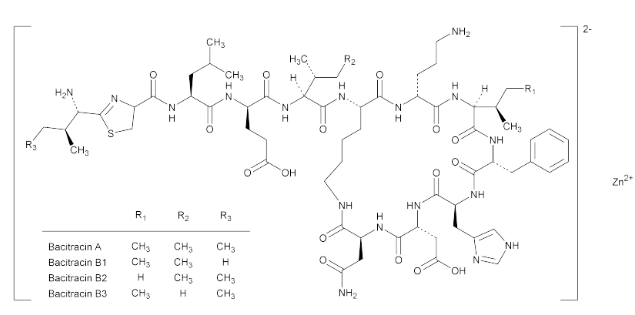
Bacitracin Zinc is the zinc complex of bacitracin, which consists of a mixture of antimicrobial polypeptides, the main components being bacitracins A, B1, B2, and B3. It has a potency of not less than 65 Bacitracin Units/mg, calculated on the dried basis. It contains not less than 4.0% and not more than 6.0% of zinc (Zn), calculated on the dried basis.
- Chemical Structure
Applications & Uses
Properties
- Physical Form
Regulatory & Compliance
- Certifications & Compliance
- Chemical Inventories
- FDA Disclaimer
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
- USP Reference Standards
- USP Bacitracin Zinc RS
Packaging & Availability
- Labelling Information
Label it to indicate that it is to be used in the manufacture of nonparenteral drugs only. Where it is packaged for prescription compounding, label it to indicate that it is not sterile and that the potency cannot be assured for longer than 60 days after opening, and to state the number of Bacitracin Units/mg. Where it is intended for use in preparing sterile dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of sterile dosage forms.
